- Methodology
- Open access
- Published:
Metadta: a Stata command for meta-analysis and meta-regression of diagnostic test accuracy data – a tutorial
Archives of Public Health volume 80, Article number: 95 (2022)
Abstract
Background
Although statistical procedures for pooling of several epidemiological metrics are generally available in statistical packages, those for meta-analysis of diagnostic test accuracy studies including options for multivariate regression are lacking. Fitting regression models and the processing of the estimates often entails lengthy and tedious calculations. Therefore, packaging appropriate statistical procedures in a robust and user-friendly program is of great interest to the scientific community.
Methods
metadta is a statistical program for pooling of diagnostic accuracy test data in Stata. It implements both the bivariate random-effects and the fixed-effects model, allows for meta-regression, and presents the results in tables, a forest plot and/or summary receiver operating characteristic (SROC) plot. For a model without covariates, it quantifies the unexplained heterogeneity due to between-study variation using an I2 statistic that accounts for the mean-variance relationship and the correlation between sensitivity and specificity. To demonstrate metadta, we applied the program on two published meta-analyses on: 1) the sensitivity and specificity of cytology and other markers including telomerase for primary diagnosis of bladder cancer, and 2) the accuracy of human papillomavirus (HPV) testing on self-collected versus clinician-collected samples to detect cervical precancer.
Results
Without requiring a continuity correction, the pooled sensitivity and specificity generated by metadta of telomerase for the diagnosis of primary bladder cancer was 0.77 [95% CI, 0.70, 0.82] and 0.91 [95% CI, 0.75, 0.97] respectively. Metadta also allowed to assess the relative accuracy of HPV testing on self- versus clinician-taken specimens using data from comparative studies conducted in different clinical settings. The analysis showed that HPV testing with target-amplification assays on self-samples was as sensitive as on clinician-samples in detecting cervical pre-cancer irrespective of the clinical setting.
Conclusion
The metadta program implements state of art statistical procedures in an attempt to close the gap between methodological statisticians and systematic reviewers. We expect the program to popularize the use of appropriate statistical methods for diagnostic meta-analysis further.
Background
Meta-analysis of diagnostic test accuracy (DTA) studies using approximate methods such as the normal-normal model has several challenges. These include poor statistical properties when sensitivity and/or specificity are close to the margins i.e. 0/1, when the sample sizes or when the number of studies are small. Moreover, the sample variance of sensitivity/specificity is a function of the sample mean and ignoring this mean-variance relationship may bias the summary estimate and its variance. Generalized linear mixed models (GLMM) [1] are therefore recommended [2]. These models are relatively complex requiring expertise both in GLMMs and statistical programming. Scientists in the fields of public health, epidemiology or clinical research often do not have advanced statistical and/or programming skills. Hence, availability and dissemination of appropriate and optimal statistical methods in a robust and user-friendly program is quintessential.
The two most commonly used statistical models for pooling of DTA data are the hierarchical summary receiver operating characteristic model (HSROC) [3] and the bivariate random-effects meta-analysis model (BRMA) [2]. The two models incorporate covariates differently though they have been shown to be equivalent when no covariates are included [4].
The proportion of total unexplained variation due to between-study heterogeneity is usually quantified using the I2 statistic by Higgins and Thompson [5]. The statistic is based on the normal-normal model and was defined for univariate meta-analysis. Therefore, in meta-analysis of DTA separate statistics for sensitivity and specificity are computed. The fact that diagnostic data sets are binomial implies that the within-study variance in sensitivity and specificity parameters is a function of the mean parameters. Hence, heterogeneity statistics based on the normal-normal model tend to underestimate the expected value of the within-study variance resulting in high values of I2. This could lead to an incorrect conclusion of very high heterogeneity [6].
Zhou and Dendukuri [6] proposed a univariate I2 statistic that accounts for the mean-variance relationship across studies. They extended the statistic to account for the correlation between sensitivity and specificity yielding a joint measure of heterogeneity. In a simulation study, they showed that their I2 statistic almost always resulted in much lower between-study heterogeneity estimates than the I2 by Higgins and Thompson [5].
On interpreting the I2, higher values indicate higher between-study heterogeneity across the studies compared to the expected within-study variability.
The reasons for the substantial heterogeneity in the null mixed-effects model can be explored by relating study level covariates to the latent sensitivity and specificity. This is called meta-regression.
There are two Stata commands for meta-analysis of DTA. The metandi [7] command fits both the HSROC and the BRMA model. Its output includes a table of the summary accuracy measures and a graph with the SROC curve, the summary point and its confidence region and prediction region. The command does not allow meta-regression.
midas [8] is another Stata command. It implements the BRMA only. It produces more graphical output; to explore goodness of fit, publication bias and other precision-related biases. The command only allows for univariate meta-regression with only one covariate and uses the I2 statistic based on the normal-normal model.
In this paper, we demonstrate a new Stata command metadta which implements the bivariate random-effects and the univariate fixed-effects model as a special case of the bivariate model. The command also allows for univariate and bivariate meta-regression. The results are reported in tables, forest plots and/or SROC plots or cross-hairs. A forest plot of relative sensitivity and specificity can be displayed when data are from comparative or paired studies. For the model without covariates, it quantifies the between-study heterogeneity using the I2 statistics by Zhou & Dendukuri [6].
Methods
Data structure
Data from DTA studies usually result from a 2 × 2 cross-tabulation of index versus reference test results (see Table 1). The data in the four cells represent the true positive (TP), false positive (FP), true negative (TN), and false negative (FN). The sum of TP and FN is the total with disease, and the sum of TN and FP the total without disease.
The command provides statistical procedures for data sets from independent, comparative and paired DTA studies.
Independent studies contribute only one 2 × 2 cross-table and each row in the data set has data from a different study.
From a comparative study, there will be two 2 × 2 cross-tables, one for the index test and the second for the comparator test. Each study contributes two rows to the data set, one for the index test and another for the comparator test. The index and the comparator test should be the same in all the studies.
Paired studies have at least a pair of the 2 × 2 cross-tables. The data for the index and comparator test is on the same row and a study can contribute more than one row to the data set. Unlike data from comparative studies, the index and the comparator tests do not need to be the same. However, it is imperative that the comparator tests are similar in order to obtain correct model estimates. The data set should include at least two index tests.
The logistic regression model
Consider a meta-analysis of K studies. For a study i (i = 1, …, K), let Yi1 be the number of true positive, Yi2 be the number of true negatives, Ni1 the total number of subjects with the disease, and Ni2 the total number of subjects without the disease.
Suppose there are Q study level covariates, the fixed-effects model is formulated as follows;
Yij~binomial (pij, Nij) for i = 1, …, K and j = 1, 2,
where pi1 and pi2 are parameters denoting the unobserved sensitivity and specificity in study i respectively. \( {\beta}_0^j \) are log-odds while \( {\beta}_1^j \) … \( {\beta}_Q^j \) are log odds ratios. \( {X}_{ij}^q \) is the value of the q’th covariate in study i for logit sensitivity(j = 1) and logit specificity (j = 2).
The random-effects model has (un) correlated random components in the mean predictor. It is expressed as follows;
where δij are the study-specific random-effects for the logit sensitivity(j = 1) and the logit specificity (j = 2). The variation in the two random effects and their correlation is represented by Σ. The structure of Σ can be any of the four variance-covariance matrices: unstructured \( \left(\begin{array}{cc}{\tau}_1^2& {\tau}_{12}\\ {}{\tau}_{12}& {\tau}_2^2\end{array}\right) \), independent \( \left(\begin{array}{cc}{\tau}_1^2& 0\\ {}0& {\tau}_2^2\end{array}\right) \), exchangeable \( \left(\begin{array}{cc}{\tau}^2& {\tau}_{12}\\ {}{\tau}_{12}& {\tau}^2\end{array}\right) \) or identity \( \left(\begin{array}{cc}{\tau}^2& 0\\ {}0& {\tau}^2\end{array}\right) \). The BRMA imposes the unstructured variance-covariance matrix. It makes the most relaxed assumption about the co-variation of the random effects but has the most number of parameters, i.e. 3 distinct parameters. Adding \( {\beta}_0^1 \) and \( {\beta}_0^2 \), it implies that there needs five parameters to be identified in the null model. Hence, at least five studies would be required to enable parameter identification. Other structures are more restrictive but require less studies (at least 3) for identifiability.
When a random effects model is fitted to the data set, a log-likelihood ratio (LR) test is conducted to compare it with the fixed effects model. The reported p-value is an upper bound of the actual p-value because this hypothesis test is on the boundary of the parameter space of the variance parameters.
The models presented above are applied when the data are from independent studies. With data from comparative or paired studies, the linear predictor is modified to account for the dependence introduced by the “repeated measurements” per study. This modification is critical in the interpretation of the random variation in the data as well as in obtaining valid model-based inference for the mean structure.
When there are more than one covariates, the fixed effects component in the linear predictor can be extended to include interaction terms between the first covariate and the remaining covariates. A LR test can then be conducted comparing the model with and without the interaction terms. This would give an answer as to whether the interaction terms are necessary in the model.
Summary tables
We report the marginal summary estimates and not the direct model parameter estimates. The marginal sensitivity and specificity are averages of the predicted probabilities from the model. They are said to be standardized to the distribution of the covariates [7, 9]. The model-adjusted probability ratios are computed as a ratio of the marginal probabilities.
Forest plot
The command presents five different confidence intervals (CI) for the study-specific sensitivity and specificity; the Wald, Wilson, Agresti-Coull, Jeffreys, and exact confidence intervals. The exact confidence intervals are displayed by default.
With data set from comparative and paired studies, the Koopman score confidence intervals [10] for the study-specific relative sensitivity and specificity are calculated.
SROC plot
Separate summary points and their confidence regions and/or prediction regions are presented when there is only one categorical covariate in the model. When the number of studies is insufficient to fit the random-effects model or when the fixed-effects model is explicitly applied, cross-hairs indicating the confidence intervals of the summary estimates are presented instead. In presence of more than one covariate, the SROC plot presents only the overall summary point and the corresponding confidence region and/or prediction region. When focus of is on the relative diagnostic accuracy i.e. when the forest plot presents the relative sensitivity and specificity, the SROC plot is not presented.
When plotting the SROC curve, the program restricts the curve to the range of the specificities in the dataset.
Software installation
The metadta command was developed in Stata 14.2. The program along with the help files and three demonstration datasets are publicly available for downloading at https://ideas.repec.org/c/boc/bocode/s458794.html. When connected to the internet, the command can be directly installed within Stata by typing ssc install metadta.
Syntax

The metadta command requires five main arguments to run. These are; tp fp fn tn indicating the four outcome variables from the 2 × 2 cross-tabulation in Table 1. The fifth argument studyid(varname) is the study identifier. The other arguments in italics are optional.
Categorical variables in the data set should be string variables otherwise, the command will treat them as continuous variables. The Stata command decode can be used to make a factor variable into a string variable. The covariates names should not contain the underscore(_) character. If present, the program terminates because the underscore character is reserved in the program. If some of the covariates names contain the underscore character, the Stata command rename can be used to give those covariates different names. The options [options foptions soptions] could be;

Once installed, typing help metadta should display the help window. The help file provides a detailed description of all the command options. Some of the options worth mentioning here include;
by varlist: allows separate but similar meta-analyses for each level of the by variable or each combination of the by varlist variables. The results are presented in separate summary tables, forest plots and SROC plots. If it is desired to perform separate analysis but present the results in one forest plot and/or one SROC plot, one should specify the options stratify and by(byvar) simultaneously. The two options by varlist: and by(byvar) should not be confused for each other.
indepvars indicates one or more variables to be used as covariates. They should be string/characters for categorical variables and/or numeric for continuous variables. The variable names should not contain underscores, it is reserved in the program.
comparative indicates whether the data supplied are from comparative studies. This option requires the first covariate specified to be categorical with two levels, one for the index and the comparator test or level.
paired indicates whether the data supplied are from paired studies. This option requires at least 11 variables (including the study identifier) in the data set in the following order tp1 fp1 fn1 tn1 tp2 fp2 fn2 tn2 index comparator.
* are options native in Stata to change the graphics aesthetics. The default plots are already visually appealing but can be optimized by soptions() and foptions() for the SROC plot and the forest plot, respectively.
Application
Example one – random-effects model with no covariates
Glas et al. [11] conducted a meta-analysis to assess the sensitivity and specificity of urine based markers such as telomerase for diagnosis of primary bladder cancer. This data set of 10 studies is provided along with the installation files.

Because the seventh study Kinoshita1997 had an estimated specificity equal to one (fp = 0), the authors needed to use a continuity correction of 0.5 to enable parameter estimation with the bivariate normal-normal model. They reported that telomerase had a sensitivity and specificity of 0.75 [95% CI, 0.66, 0.74] and 0.86 [95% CI, 0.71, 0.94] respectively. They concluded that telomerase was not sensitive enough to be recommended for daily use.

Apart from the required main arguments, we also specified the option model (random) to request for the random-effects model. This option is redundant since the command fits the random-effects by default. When the program detects that the number of studies is less than 3, the fixed-effects model is fitted instead. dp(2) requests the results of all estimates to be displayed with 2 decimal places (except the p-values for which the decimals places are fixed at 4). The options in soptions() and foptions() refined the appearance of the forest and SROC plots.
The first part of the output displays the symbolic representation of the fitted model, the number of observations and the number of studies in the meta-analysis as shown below;

The next part of the output presents the heterogeneity statistics. This table can be suppressed by the option nohtable. By default, the unstructured covariance matrix is imposed. The correlation (rho) between sensitivity and specificity on the logit scale is − 1. There is more heterogeneity in specificity (σ2 = 3.32, I2 = 60.29%) than in sensitivity σ2 = 0.18, I2 = 50.62 % ).
Despite presence of heterogeneity in both dimensions, it may be surprising that the bivariate I^2 = 0.02. This is because the generalized between-study variance goes to zero with (nearly) perfect correlation (rho = − 1.00), and the lower the bivariate I^2. The generalized between-study variance was < 0.0001. It summarizes the variance in both logit sensitivity and specificity while accounting for the correlation between them.
The p-value of the LR test comparing the fitted random-effects to a fixed-effects model is < 0.0001. This indicates that the random-effects is a better fit to the data. The test has three degrees of freedom since the unstructured covariance matrix has 3 parameters.

sumtable (all) requested for all available summary tables, i.e. summary estimates on the log odds and the probability scale. These are presented as follows;

The mean logit sensitivity and specificity are 1.19 [95% CI, 084, 1.55] and 2.34 [95% CI, 1.10, 3.58]. The p-values from testing whether the logit sensitivity or logit specificity is 0 are both < 0.01. Thus the logits are significantly different from zero.
The second table presents the same summary statistics but on the probability scale. The standard errors, the z-statistic and the p-values are reported on the logit scale. Translated in the probability scale, the p-values are from testing whether the mean sensitivity/specificity is 0.5. If needed, one can use the delta method to compute the standard errors on the probability scale.
The pooled sensitivity and specificity of telomerase in urine as a tumour marker for the diagnosis of primary bladder cancer was 0.77 [95% CI, 0.70, 0.82] and 0.91 [95% CI, 0.75, 0.97] respectively. Our results are different from the original publication because we use the logistic-normal model while they used the normal-normal model.
The third table below presents the study-specific and summary sensitivity and specificity and their corresponding 95% exact CI.

Figures 1 and 2 (left) presents the forest and the SROC plots respectively. The program preserves the order in the data set. Say it is preferred to order the studies by year of publication, a variable with the year of publication (say year) should be included in the data set. The option sortby(year) then instructs the program to re-order the data set.
Model comparison - covariance structures
To impose a different covariance structure, say independence and select the most parsimonious model we proceeds as follows;
-
1.
First restore the model estimates by typing estimates restore metadta_modest (the estimates of the current model are always stored as metadta_modest). Once restored, use the command estat ic to display the Akaike information criteria (AIC) and Bayesian information criteria (BIC) [12]. The output is;
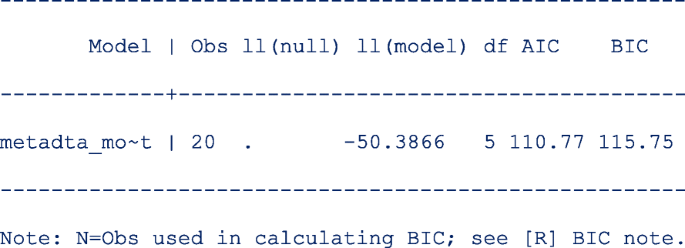
-
2.
Use the command estimates store to store the estimates for later use under a different name, say unstructured. i.e. estimates store unstructured.
-
3.
Fit a new model imposing independence between logit sensitivity and logit specificity with the option cov(independent).
-
4.
Repeat step 1 above to be able to display the information criteria. The output is;
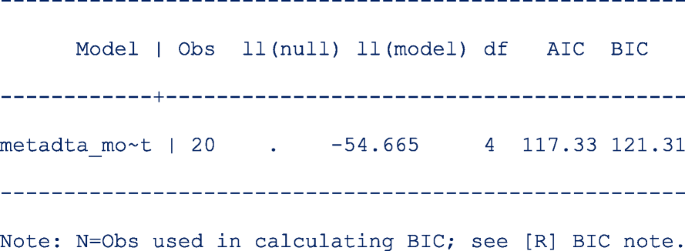
The models compared using the information criteria do not need to be nested but should use the same data. The model with a smaller information criterion fits the data better. From the output in steps 1 and 4, the model with the unstructured covariance matrix fits the data better since both the AIC and the BIC are lower.
Sometimes the AIC and BIC can give conflicting conclusions. In this example, both give the same conclusion. The difference between AIC and BIC is in measuring the model complexity. Model complexity is measured either as 2*q or ln (K)*q, where q is the number of parameters estimated in the model and K is the number of observations in the data set. Explicitly,
AIC = − 2 x ln (likelihood) + 2 x q.
BIC = − 2 x ln (likelihood) + ln(K) x q.
By overweighting the model complexity, the BIC is more conservative than AIC.
Implication of assuming the independence covariance structure
The pooled sensitivity and specificity under the independence assumption is 0.77 [95% CI, 0.70, 0.82] and 0.90 [95% CI, 0.75, 0.97]. The pooled estimates are very similar to those from the first model.
However, the heterogeneity statistics are much more different; logit sensitivity (σ2 = 0.15, I2 = 46.81%) and logit specificity (σ2 = 2.75, I2 = 65.01%). The estimate for the generalized variability is much higher (σ2 = 0.43, I2 = 56.11%) because there is (assumed) no correlation.
When the assumed covariance structure is far from ‘correct’, the estimates for the mean always tend to be consistent. However, the confidence intervals, confidence region and prediction region might be wider. In Fig. 2 (right), assuming no correlation between the logit sensitivity and the logit specificity yields wider regions.
Example two – random-effects meta-regression
Arbyn et al. [13] published a meta-analysis on the accuracy of HPV testing on self-collected versus clinician-collected samples. In the review, they sought to find whether a HPV test on a vaginal self-sample was as accurate as on a cervical sample taken by a clinician to detect cervical precancer (cervical intraepithelial neoplasia of grade 2 or worse [CIN2+]). metandi [7] was used to generate the pooled absolute sensitivity and specificity and metadas [14] was used to obtain the relative sensitivity and specificity (self-sample vs clinician sample) by the test amplification method (signal or target amplification).
The studies included in the meta-analysis had been conducted in three clinical settings: 1) cervical cancer screening, 2) testing of high-risk women, and 3) colposcopy, where women were referred to because of previous positive screening results.
Other information on the study participants, name of the test used, the sampling device were recorded also.
We use a sample of the published data set where studies applied the same test on a self-sample and a clinician-sample from the same women (comparative studies). The first 10 of the 60 observations are as below;

where sample, setting and ta are categorical (characters/string) covariates each with two values. sample is clin or self in the clinician-sample and self-sample respectively. Year indicates the year of study publication. setting identifies the clinical setting of the study with values screening or follow-up. ta is TA for a target amplified test or SA for a signal amplified test.
Exploratory analysis
We investigate whether the absolute sensitivity and specificity of the self- and clinician-collected sample differ by the test amplification method and the setting of the study. To do this, we fit four models for each combination of the categories in setting and ta with sample as a covariate.
The command preserves the order of the data and therefore, the values in each categorical variable are decoded based on the first-come-first-assignment. The Stata command gsort sorts the data alphabetically such that the base level for sample is clin and the second level is self.

The values in the categorical variables can decoded based on the alphabetic order (A to Z) while still preserving the order in the data set with the option alphasort. The first value of the categorical covariates used in the model are assigned the base levels.
The code to fit the first model is as follows;

The option noitable suppresses the table with the study-specific estimates, and sumtable (abs rr) requests to display the absolute and relative specificity and relative sensitivity only (the summary table of the log odds will be suppressed). In this analysis, we are not interested in the SROC plot and suppress its display with the option nosroc.
From the output below, there are 6 observations from 3 studies in the meta-analysis.

In this model, the base level in sample is clin.

The next part of the output below displays the linear predictor representation of two simpler models fitted to the data set for model comparison. The fitting of the additional models could take some time especially in more complex or larger models. If not necessary, they can be skipped with the option nomc. The two simpler models leave out the sample term in each of the two predictor equations.

In meta-regression, the I2 statistics are not calculated. From the output below, there is more heterogeneity on the logit sensitivity (Tau.sq. = 0.56) than on the logit specificity (Tau.sq. = 0.06). The generalized I2 is even less (Tau.sq. = 0.03) after accounting for the correlation (rho = 0.13) between the logits. Compared to the model with fixed study effects, the model with random study effects fits the data better (p = 0.0018).

The table below shows the pooled sensitivity and specificity of signal amplified HPV tests on self- and clinician-samples in the follow-up setting:

The next table (below) shows the pooled relative sensitivity and specificity of signal amplified HPV tests on self- vs clinician-samples in follow-up setting;

From the output above, the signal amplified tests on self and clinician samples have similar sensitivity (Rel Ratio = 0.92 [95% CI: 0.81, 1.04]) and specificity (Rel Ratio = 0.91 [95% CI, 0.81, 1.02]) in the follow-up setting.
The output below shows the model comparisons results. The results indicate that the model without sample on the linear predictor for logit sensitivity or logit specificity is more parsimonious (p-values are > 0.05 in both cases). The conclusion here from the LR test is essentially the same as from the table with the relative diagnostic accuracy estimates.

We fit the other three models by changing if ta==“SA” & setting==“follow-up” to include the other combinations of the test amplification method and the setting. Figure 3 presents the forest plots from the four fitted models. From the forest plots, the pooled specificity is consistently lower in follow-up settings (range 50–63%) and substantially higher values in the screening setting (range 84–88%) suggesting that the absolute accuracy differed by settings. Our interest however, is in answering whether a HPV test on a vaginal self-sample is as good as on the cervical sample taken by a clinician.
Confounding effects
To formally assess the differences by setting, we enter a second covariate into the model. In another instance, we do the same for the test amplification method ta. In the command, we indicate that the studies in the data set are comparative with the option comparative and request for a forest plot of the relative specificity and relative sensitivity with option outplot (rr) in foptions(). The SROC plot is automatically not generated when the option outplot (rr) is specified.
In Fig. 4, we observe that the relative sensitivity of signal amplified tests on self- vs clinician- sample is consistently lower than unity irrespective of setting (top left). In contrast, the relative sensitivity of target amplified tests on self- vs clinician-sample consistently includes unity (bottom left) in the screening and follow-up setting. The pooled relative specificity show limited variation by setting.
The findings indicate that it is reasonable to report the “pooled” relative accuracy estimates without regard to setting for a given method of test amplification.
Including the interaction terms
The plots in Figs. 3 and 4 suggest that the setting and the test amplification method both significantly influence the absolute accuracy but that the setting has a minor influence on the relative accuracy. To formally examine how setting and ta modify the sensitivity and specificity for CIN2+ of HPV testing on self- and clinician-sample we include the covariates ta and setting in the model. We also include the interactions terms between sample and ta and between sample and setting by specifying the option interaction(sesp). This option specification instructs the program to add interaction terms on both the linear predictors for logit sensitivity and specificity.

The dataset in the meta-analysis comprised 60 observations from 28 studies.

We requested for the diagnostic accuracy estimates with the option sumtable(rr) though we do not present the table here. Controlling for the method of test amplification, the relative sensitivity of HPV testing on self- vs. clinician-sample in the screening and follow-up settings are 0.91 [95% CI: 0.86, 0.95] and 0.92 [95% CI: 0.86, 0.98] respectively. The confidence intervals over-lap suggesting that there might be little or no difference in the pooled relative sensitivities. Similarly, controlling for the clinical setting, the relative sensitivity in target-amplified and signal-amplified tests are 0.82 [95% CI: 0.76, 0.89] and 0.98 [95% CI:0.95, 1.01] respectively. The confidence intervals do not overlap suggesting differences by test amplification method. The relative specificity can be interpreted in a similar manner.
Wald-type tests for non-linear hypotheses were conducted to formally test whether the relative sensitivities and relative specificities were similar in all settings and test amplification methods. The results are displayed as follows;

From the output above, each hypothesis test has one degree of freedom because there is only one contrast examined, e.g. for setting, the contrast is RR.screening = RR.follow-up.
The results indicate that after controlling for the test amplification method, the relative sensitivities were similar (p = 0.7049) in the two clinical settings. In contrast, the pooled relative sensitivities were different (p = 0.0001) between the two test amplification methods after controlling for the clinical setting. Furthermore, there were no differences in the pooled relative specificities by clinical setting (p = 0.4799) or by test amplification method (p = 0.6909) after controlling for the type of test and the clinical setting respectively.
We also tested whether the interaction terms were significant by leaving out one interaction term in each of the predictor equation at a time. The output below indicates that neither of the two interaction terms in the predictor equation for logit specificity are significant.

Fitting a simpler model
The simpler model without interaction terms in the predictor equation for logit specificity is fitted to the data set with the option interaction(se). The option instructs the program to add the interaction terms only in the predictor equation for logit sensitivity.
Before running the command, we restore and store the model estimates under a different name (say full) for use later to compare the current model (interaction(sesp)) with the next model (interaction(se)).

The predictor equations for the model with interaction terms only on the logit sensitivity are as follows;

Other simpler models leaving out the interaction terms or the main term from the predictor equation for the logit sensitivity and the logit specificity respectively are also fitted to the data. The model comparison results are as follows;

From the output above, leaving out ta*sample (p = 0. 3800) or setting (p = 0. 3700) from the linear predictor of the logit sensitivity and logit specificity respectively, would yield a more parsimonious model.
We restore and store the estimates under the name reduced and request for the AIC and BIC of the current model.

Both the AIC (reduced = 907.6009, full = 908.0111) and BIC (reduced = 943.8383, full = 949.8235) indicate that the reduced model fits the data set slightly better.
As mentioned earlier from Leave-one-out LR Tests: Model comparisons, the reduced model could be improved further by removing more terms from the predictor equations. However, the metadta program is not flexible to fit a model without ta*sample while keeping setting*sample or a model that includes the interaction terms on the logit sensitivity but leave out the main term for setting in the predictor equation of the logit specificity. Nonetheless, this “fine-tuned” model can be fitted outside metadta via the native Stata command meqrlogit.
Figure 5 displays the forest plot from the full (on the left) and the reduced model (on the right). There are differences in the estimates for the pooled relative specificity but not for the pooled relative sensitivity.
The pooled relative diagnostic accuracy estimates from the reduced model in Fig. 5 (right) are very similar to the “stratified” meta-regression results presented in Fig. 4.
Discussion
This tutorial demonstrated some of the capabilities of metadta to perform meta-analysis and meta-regression of DTA studies in Stata. Random-effects models with and without covariates were fitted using logistic regression. The model-adjusted pooled absolute and relative diagnostic accuracy were presented in tables and graphically in forests and/or SROC plots.
We developed metadta to provide advanced statistical procedures for data sets from independent, comparative and paired DTA studies. We encourage users of our program to explore the help file and run the demonstration examples to further familiarize with metadta.
With metadta, we expect to close the gap between expert methodological statisticians and systematic reviewers and to boost the use of more appropriate methods for meta-analysis and meta-regression of DTA studies even further.
Availability of data and materials
The metadta program was developed in Stata 14.2. The code, the help files used herein are publicly available for download at https://ideas.repec.org/c/boc/bocode/s458794.html.
Change history
27 September 2022
A Correction to this paper has been published: https://doi.org/10.1186/s13690-022-00953-9
Abbreviations
- SROC:
-
Summary receiver operating characteristic
- HPV:
-
Human papillomavirus
- DTA:
-
Diagnostic test accuracy
- GLMM:
-
Generalized linear mixed models
- HSROC:
-
Hierarchical summary receiver operation characteristic model
- BRMA:
-
Bivariate random-effects meta-analysis
- TP:
-
True positive
- FP:
-
False positive
- FN:
-
False negative
- TN:
-
True negative
- AIC:
-
Akaike information criterion
- BIC:
-
Bayesian information criterion
- CIN2 +:
-
Cervical intraepithelial neoplasia of grade 2 or worse
- TA:
-
Signal-amplification
- SA:
-
Test-amplification
References
Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88(421):9.
Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006;59(12):1331–2. https://doi.org/10.1016/j.jclinepi.2006.06.011.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84. https://doi.org/10.1002/sim.942.
Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8(2):239–51. https://doi.org/10.1093/biostatistics/kxl004.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Zhou Y, Dendukuri N. Statistics for quantifying heterogeneity in univariate and bivariate meta-analyses of binary data: the case of meta-analyses of diagnostic accuracy. Stat Med. 2014;33(16):2701–17. https://doi.org/10.1002/sim.6115.
Harbord RM, Whiting P. Metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J. 2009;9(2):211–29. https://doi.org/10.1177/1536867X0900900203.
Dwamena AB, Sylvester R, Carlos RC. midas: meta-analysis of diagnostic accuracy studies. Available from: https://fmwww.bc.edu/repec/bocode/m/midas.pdf. 2009. p. 2–25. Accessed 8 Feb 2017.
Muller CJ, Maclehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43(3):962–70. https://doi.org/10.1093/ije/dyu029.
Koopman PAR. Confidence intervals for the ratio of two binomial proportions. Biometrics. 1984;40(2):513. https://doi.org/10.2307/2531405.
Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PMM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol. 2003;169(6):1975–82. https://doi.org/10.1097/01.ju.0000067461.30468.6d.
Aho K, Derryberry D, Peterson T. Model selection for ecologists: the worldviews of AIC and BIC. Ecology. 2014;95(3):631–6 Available from: http://www.esajournals.org/doi/full/10.1890/13-1452.1.
Arbyn M, Verdoodt F, Snijders PJF, Verhoef VMJ, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–83. Available from:. https://doi.org/10.1016/S1470-2045(13)70570-9.
Takwoingi Y, Deeks JJ. MetaDAS: A SAS macro for meta-analysis of diagnostic accuracy studies. Quick reference and worked exampleVersion 1.3.=. Available from: http://srdta.cochrane.org/. Accessed 30 July 2010.
Acknowledgements
We are grateful to the two reviewers for all their valuable comments that helped improve the paper considerably.
Funding
Financial support was received from Horizon 2020 Framework Programme for Research and Innovation of the European Commission, through the RISCC Network (Grant No. 847845), the Belgian Cancer Centre, European Society of Gynaecological Oncology (ESGO), National Institute of Public Health and the Environment (Bilthoven, the Netherlands) and the German Guideline Program in Oncology (German Cancer Aid project).
Author information
Authors and Affiliations
Contributions
VNN wrote the metadta program, analysed the data and drafted the manuscript. MA conceptualized the project, tested the program, and edited the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: From the section Exploratory analysis, the correct codes have been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nyaga, V.N., Arbyn, M. Metadta: a Stata command for meta-analysis and meta-regression of diagnostic test accuracy data – a tutorial. Arch Public Health 80, 95 (2022). https://doi.org/10.1186/s13690-021-00747-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-021-00747-5







